Diverse Voices in International Security: NTI’s Gabby Essix on Promoting Diversity, Equity, and Inclusion in the Biosecurity Field
NTI | bio Program Officer Gabby Essix discusses diversity, equity, and inclusion (DEI) in the biosecurity field.
Atomic Pulse
This blog post was written by NTI summer interns Angela Kellett and Emily Phua. Kellett, who is working with the Communications team, received her Bachelor of Science degree in Journalism and Master of Arts degree in International Relations, both from St. John’s University. Phua, who is working with NTI | bio is in her third year at American University, working towards her B.A. in biology and a Spanish translation certificate.
Imagine scientists researching a virus in a laboratory setting. Now imagine the virus being released from the lab, by accident or on purpose, into the general population.
We know that a virus can pose a serious risk to society if released from the safety of a lab. Just the news of a release could incite panic; the reality could be a global pandemic. Given the stakes, how do we still advance life science research, while reducing the risk of misapplying the knowledge gained? This idea is at the heart of NTI | bio’s work and the key question to be answered by those entering the 2021 Next Generation for Biosecurity Competition.
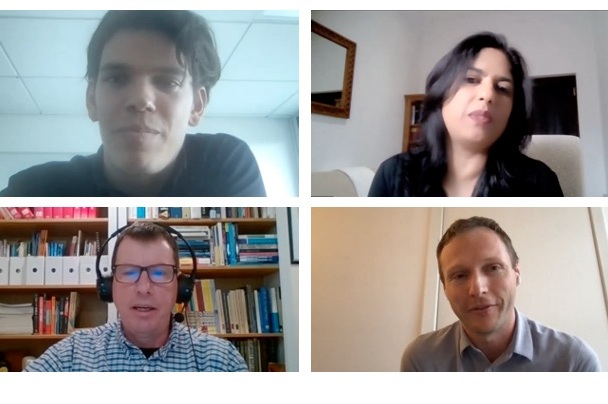
Applicants are given the difficult task of answering these questions: “What life science should not be conducted, if any? Should red lines in life science research be drawn? If so, by whom?” NTI, in partnership with the Next Generation Global Health Security (GHS) Network, hosted a 2021 Next Generation for Biosecurity Competition webinar in June to explore these questions. It was moderated by former NTI Program Officer Katherine Budeski, Argentina Country Coordinator Mayra Ameneiros, and Europe Coordinator and 2020 Next Generation for Biosecurity Competition winner Jonas Sandbrink. The webinar provided an overview of this year’s questions and the qualifications required to compete in the competition.
Red Lines in Life Science Research
Panelists included R. Alta Charo, J.D., the David A. Hamburg Distinguished Fellow at NTI; Dr. Megan Palmer, executive director of Bio Policy and Leadership Initiatives at Stanford University; and Dr. Claire Standley, Assistant Research Professor for the Department of International Health and Security at Georgetown University.
To solicit opinions without generating direct answers to the question of the competition, moderators asked the panelists whether there should be red lines in life science research. The prompt for the competition “requires looking far beyond the bio-sciences [and] to look at the history of red-lines in other contexts,” Palmer said. “There is an opportunity, I think, within the answers here to look beyond biology. But it is also important to look within the past debates around biotechnology, in particular.” She said participants should explore what those red lines are, the history, and how they have shifted over time.
Asked whether she thinks red lines exist, Charo focused on the distinction between regulations related to substance and those related to process. Substantive regulations list what can and cannot be done, while process regulations provide a set of criteria to evaluate particular cases.
Charo emphasized that there is no such thing as global law governing life science research, as there is no international body responsible for regulating it. And even if there was an agreement between nations, it could be complicated by real differences between cultures regarding what is considered acceptable.
Requirements for the Next Generation for Biosecurity Competition
The Next Generation GHS Network focuses on promoting development of a diverse global health security workforce. Their mission is to foster a culture of mentoring that empowers individuals to be future leaders. Competition participants are required to create teams of three individuals from at least two different countries/regions, allowing them to work with others who may live across the world. The conglomerate of backgrounds, languages, and cultures creates diverse perspectives to seek out answers to the complex question.
The Next Generation GHS Network has more than 1,000 active members from more than 45 countries, and competition participants must be part of the network. They also are responsible for finding a mentor within the network, and they must be enrolled in an academic institution or have less than five years of professional experience.
Winners of the 2021 competition will have their paper published on the NTI website and will be able to present their work at a side-event at the 2021 Biological Weapons Convention Meeting of States Parties in Geneva (travel pending due to the ongoing pandemic).
The deadline to apply for the 2021 Next Generation for Biosecurity Competition is July 28, 2021. Participants should send their submissions in pdf format to [email protected]. Winners will be announced in October 2021.
Sign up for our newsletter to get the latest on nuclear and biological threats.
NTI | bio Program Officer Gabby Essix discusses diversity, equity, and inclusion (DEI) in the biosecurity field.
Today, leaders preparing for the BWC’s Ninth Review Conference in August are exploring whether the global community can leverage increased attention and political will to strengthen the BWC by building mechanisms that increase transparency and trust with the goal of reducing the risk of global catastrophic biological events. A particular aim is determining whether there are effective and politically viable ways to enforce the treaty.
NTI | bio Senior Program Officer Dr. Aparupa Sengupta on why efforts to strengthen global biosafety and biosecurity must prioritize diversity and inclusion.